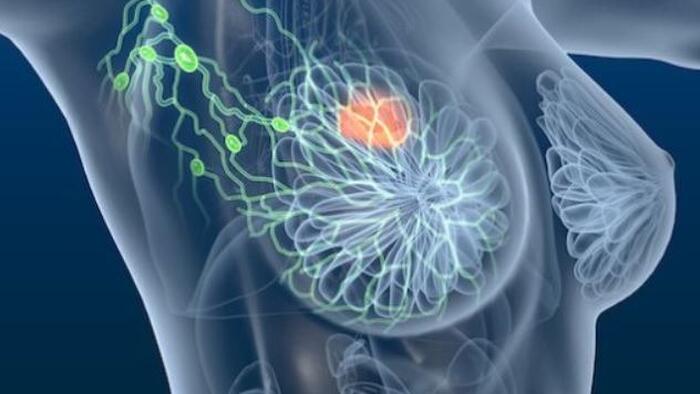
The FDA has approved Inluriyo (imlunestrant), an oral therapy for advanced breast cancer that targets estrogen receptor-1 mutations. A phase 3 clinical trial found that patients receiving the treatment had improved progression-free survival compared to those on a different investigational regimen.
FDA Approves New Therapy For Advanced Breast Cancer
Key Takeaways:
- The FDA approved this estrogen receptor antagonist on Sept. 25.
- Inluriyo is indicated for advanced or metastatic breast cancer with ER-1 mutations.
- Clinical trial participants saw a jump in progression-free survival from 3.8 to 5.5 months.
- Adverse events include abdominal pain and cardiac arrest, with a warning for fetal harm.
- Once-daily dosing is priced at $22,500 per 28 days and will be available in the coming weeks.
Overview of the Approval
The U.S. Food and Drug Administration has granted approval to a new oral therapy known as imlunestrant, or Inluriyo, offering a promising option for individuals with metastatic or advanced breast cancer. This decision, made on September 25, specifically addresses patients whose cancer is driven by mutations in the estrogen receptor-1 (ER-1) gene.
Mechanism of Action
Inluriyo functions as an estrogen receptor antagonist. ER-1 mutations, found in certain breast cancers, can cause the cells to grow and spread more rapidly. By binding to the receptor and blocking its activity, this therapy helps slow or halt cancer progression.
Clinical Trial Insight
A pivotal phase 3 trial enrolled 874 participants to evaluate the treatment’s efficacy. Notably, the group receiving imlunestrant showed better median progression-free survival—5.5 months compared to 3.8 months in a different investigational regimen. Dr. Komal Jhaveri, clinical director of early drug development at Memorial Sloan Kettering Cancer Center and principal investigator of the trial, stated that the therapy provides “a meaningful alternative treatment option for this patient population.”
Safety Considerations
According to trial data, adverse events among those taking imlunestrant included abdominal pain and, in some cases, cardiac arrest. The label also warns of potential fetal harm if taken during pregnancy. Patients are advised to discuss any concerns or prevailing health conditions with their healthcare providers.
Cost and Availability
Eli Lilly expects to make Inluriyo available in the United States within the next few weeks. The list price for the recommended once-daily 400 mg dose (two 200 mg tablets) stands at $22,500 per 28 days. While pricing may vary depending on insurance coverage and financial support programs, experts note that the oral formulation could prove simpler to manage for many patients.
Expert Perspectives
Jacob Van Naarden, executive vice president at Eli Lilly and president of Lilly Oncology, expressed gratitude for the individuals and teams that contributed to the research, emphasizing the potential for a “more manageable” treatment journey. With the FDA’s approval, the hope is that Inluriyo will provide new avenues of care for those living with ER-1 mutation-positive advanced breast cancer.