Arcutis Biotherapeutics has enrolled the first child in an open-label Phase II clinical trial to evaluate the efficacy of Zoryve (roflumilast) cream 0.05% for treating atopic dermatitis in infants. The INTEGUMENT-INFANT trial represents a significant step toward addressing a prevalent inflammatory skin condition among young children.
First child enrolled in Arcutis’ trial of atopic dermatitis cream
Key Takeaways:
- Arcutis Biotherapeutics has begun a Phase II trial for atopic dermatitis treatment in infants.
- The first infant participant has been enrolled in the INTEGUMENT-INFANT trial.
- The trial is testing the efficacy of roflumilast cream 0.05% (Zoryve).
- Atopic dermatitis is a common inflammatory skin condition in children and infants.
- The open-label trial allows for transparent observation of treatment effects.
Introduction
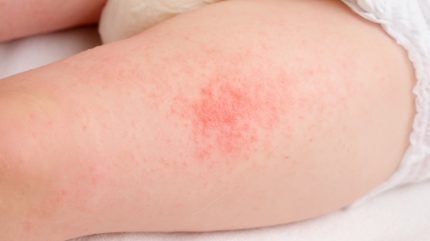
Atopic dermatitis, a prevalent inflammatory skin condition among children and infants, affects countless young patients worldwide. Characterized by itchy and inflamed skin, the condition poses significant discomfort and challenges for infants and their caregivers.
Trial Initiation by Arcutis Biotherapeutics
In a notable advancement, US-based biotechnology company Arcutis Biotherapeutics has enrolled the first child in an open-label Phase II clinical trial. This marks the beginning of their investigation into the efficacy of Zoryve (roflumilast) cream 0.05% as a treatment for atopic dermatitis in infants.
Details of the INTEGUMENT-INFANT Trial
The study, named the INTEGUMENT-INFANT trial, aims to assess how effectively roflumilast cream can address the symptoms of atopic dermatitis when applied to infants. As an open-label trial, both the researchers and participants are aware of the treatment being administered, which facilitates transparent monitoring of its effects.
Potential Impact on Atopic Dermatitis Treatment
The initiation of this trial is significant for the medical community and families affected by atopic dermatitis. If successful, the trial could lead to a new treatment option that alleviates the symptoms of this common skin condition in infants, improving their quality of life.
Conclusion
As the INTEGUMENT-INFANT trial progresses, it holds promise for infants suffering from atopic dermatitis. Arcutis Biotherapeutics’ efforts may pave the way for more effective management of the condition, offering hope to many families seeking relief for their children.